Shanghai CareFix Medical Instrument Co., Ltd
" Care for life, Health Fixed" as our mission
Shanghai CareFix Medical Instrument Co.,Ltd., abbreviated as "Health Fixed", owns the registered trademark CareFix®. Founded on June 24, 2009, the company has a registered capital of 15 million yuan and a total investment exceeding 70 million yuan.
It is a professional enterprise engaged in the research, development, design, production, sales, and technical services of orthopedic medical equipment. The manufacturing facility is located in the state-level Shanghai Songjiang Economic and Technological Development Zone (self-owned factory).
Year Founded
Exp. Country
Serving top-tier hospitals
Intellectual Property Right Certificate
Agents
With over 16 years of clinical experience, we focus on the development of complex trauma repair and orthopedic technology systems
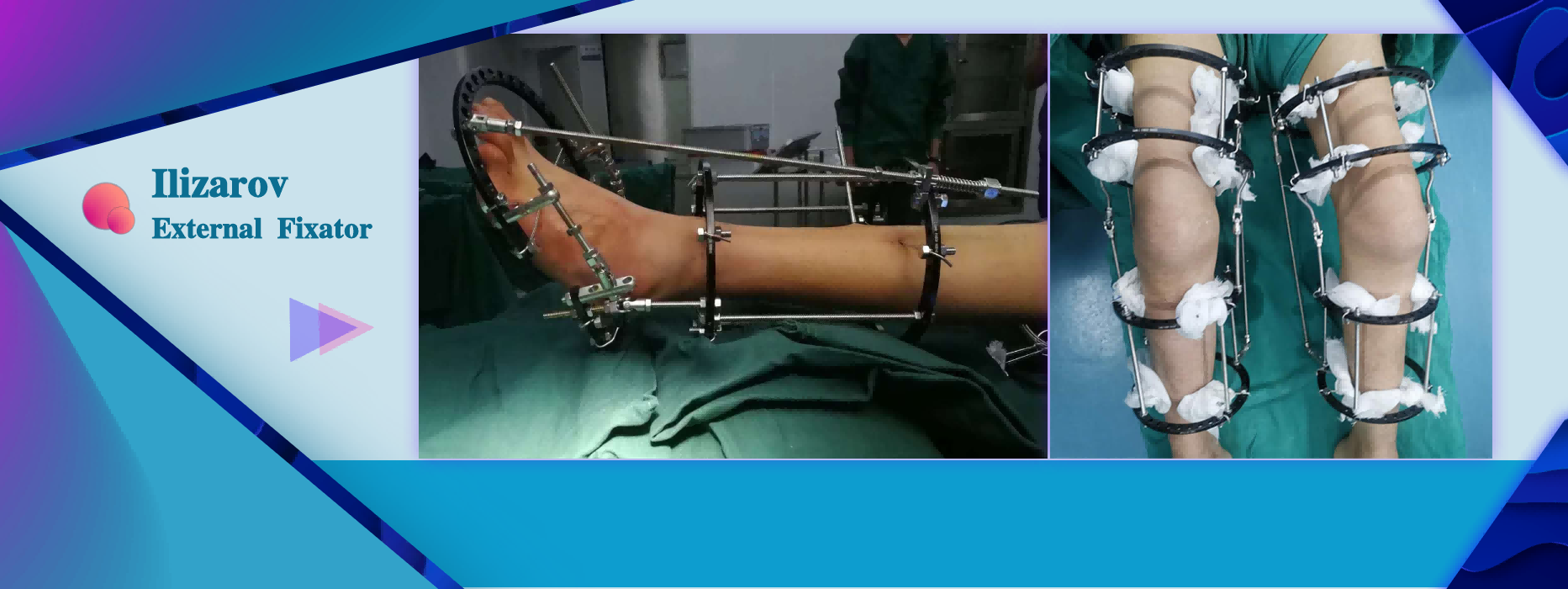
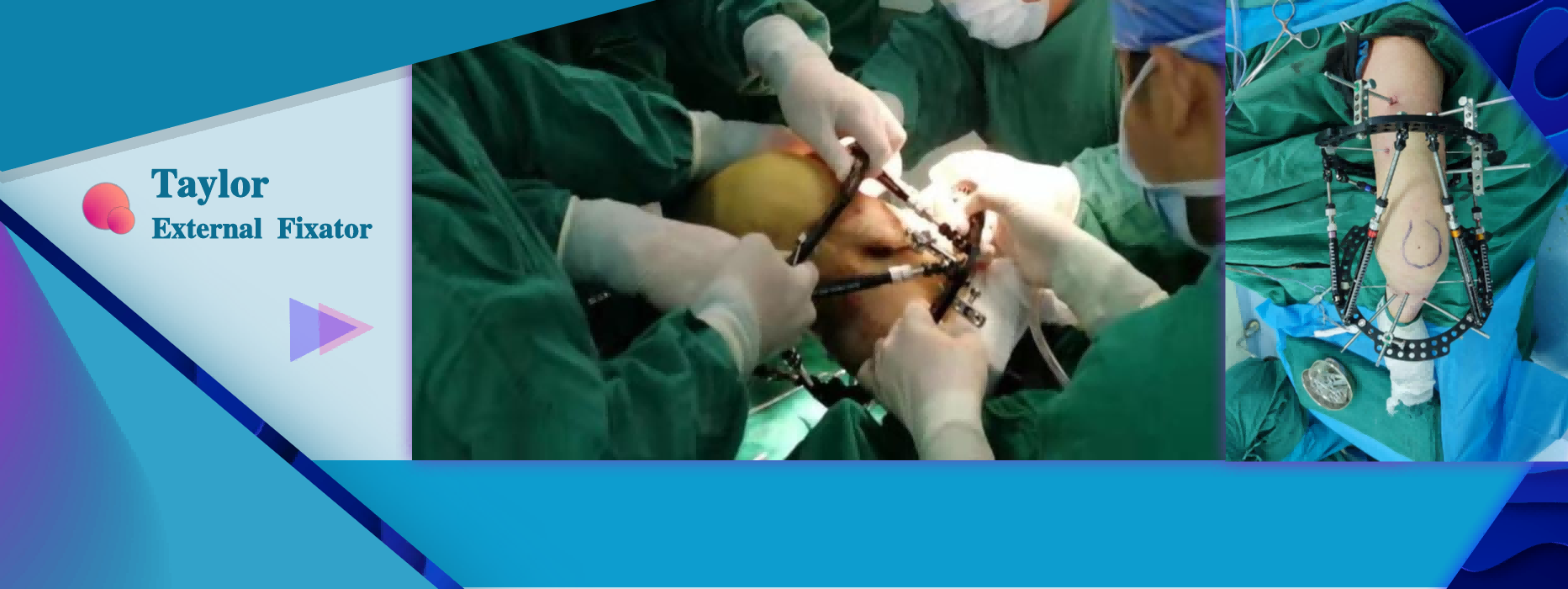
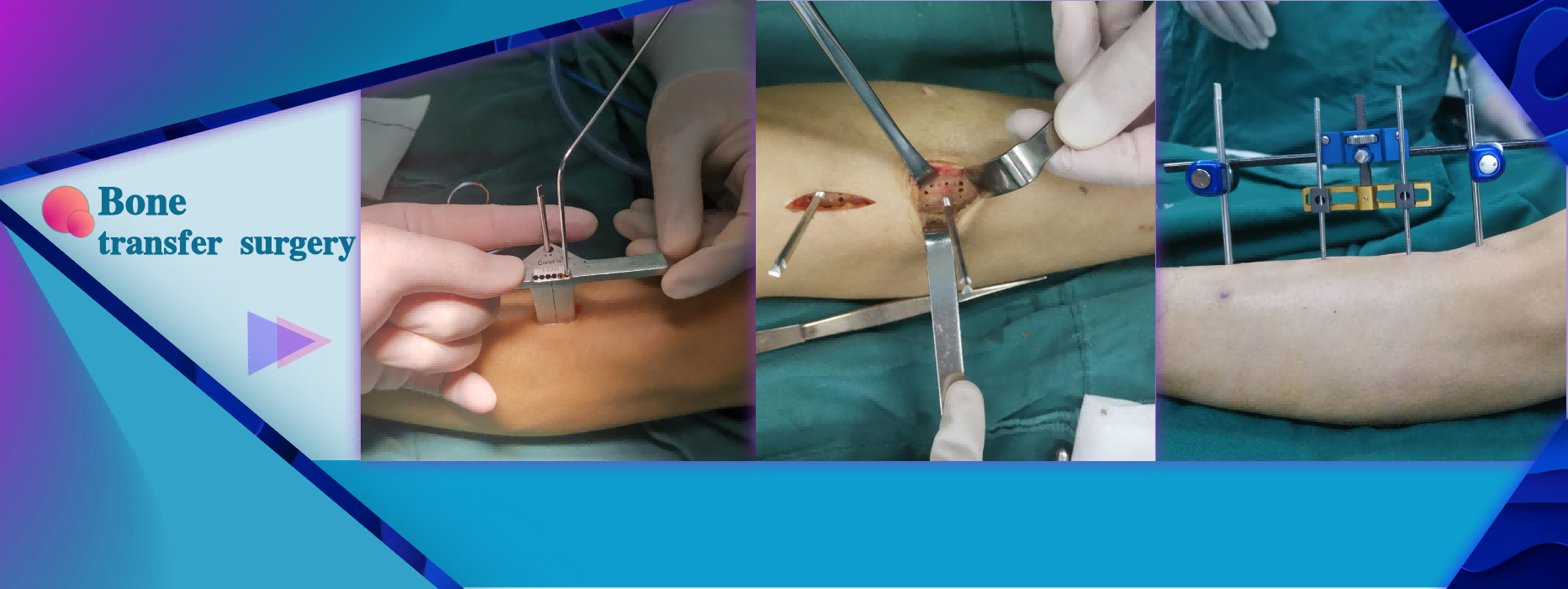
Limb extension surgery Limb reconstruction surgery Bone deformity correction surgery
More InformationLimb lengthening surgery Fracture repair surgery Limb reconstruction surgery
More InformationDiabetic lower limb ischemia (Diabetic foot) Arteriosclerosis Obliterans (ASO) Thromboangiitis Obliferans (TAO)
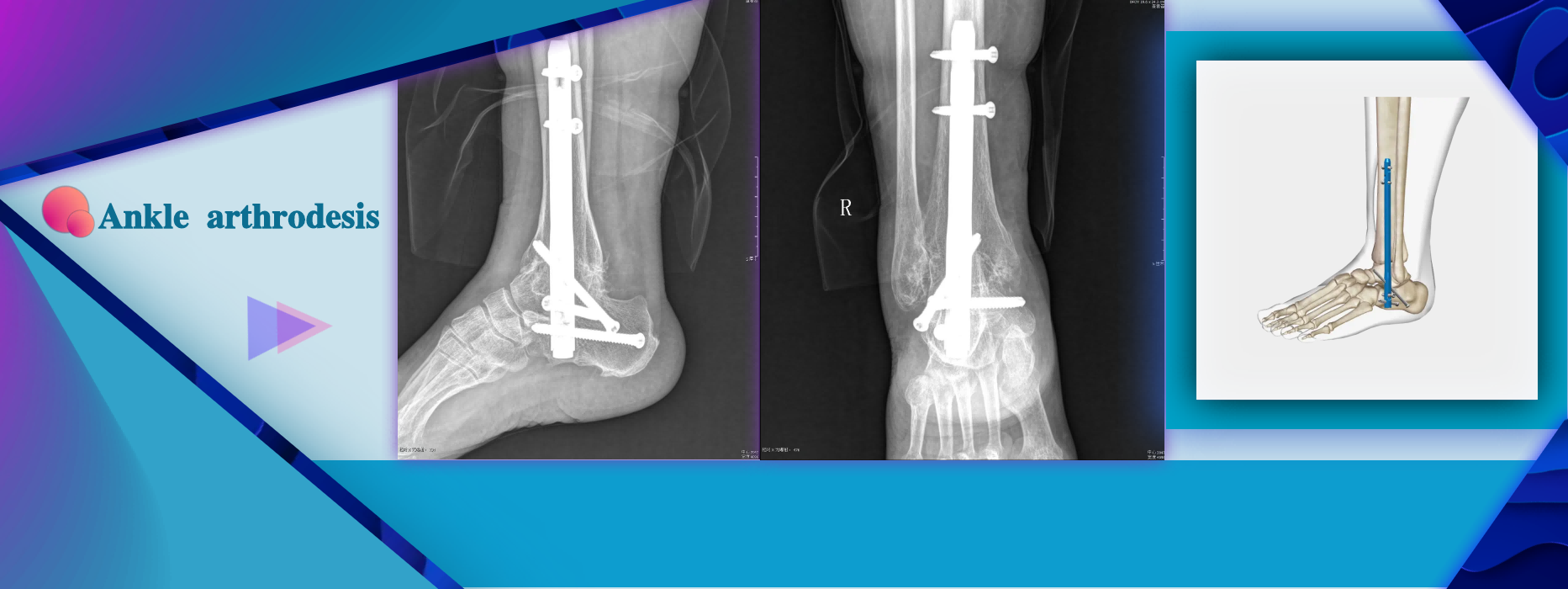
More InformationSevere foot/ankle deformity Arthrosis of ankle and subtalar joints(Traumatic,Rheumatologic) or psderoartthrosis Instability or bone infects after tumor resection Avascular necrosis of the ankle and subtalar joints Failed total ankle replacement or ankle fusion Distal tibial fracture nonunion Severe deformity secondary to neuromuscular ostero- arthropathy (Charcot's foot)
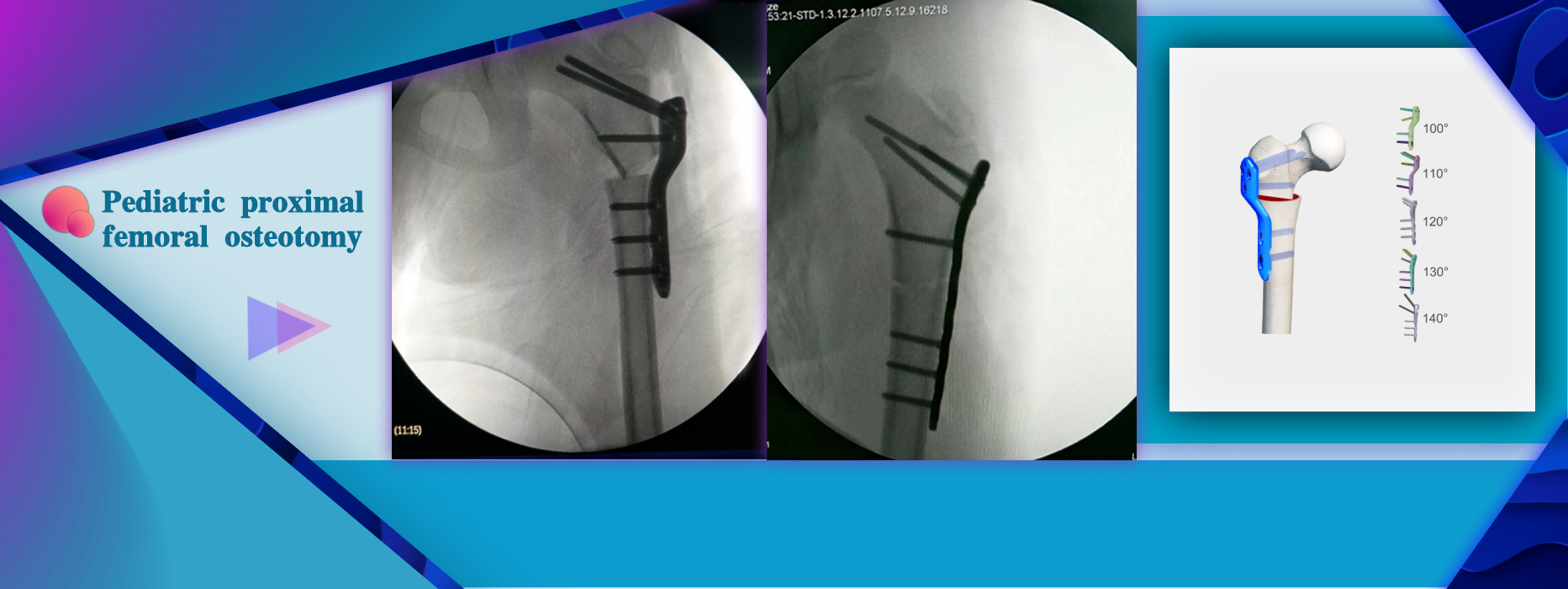
More InformationTreatment for DDH: Proximal Femoral Osteotomy Indication:Inverted, valgus, and rotational osteotomy or fracture fixation of the proximal femur in children
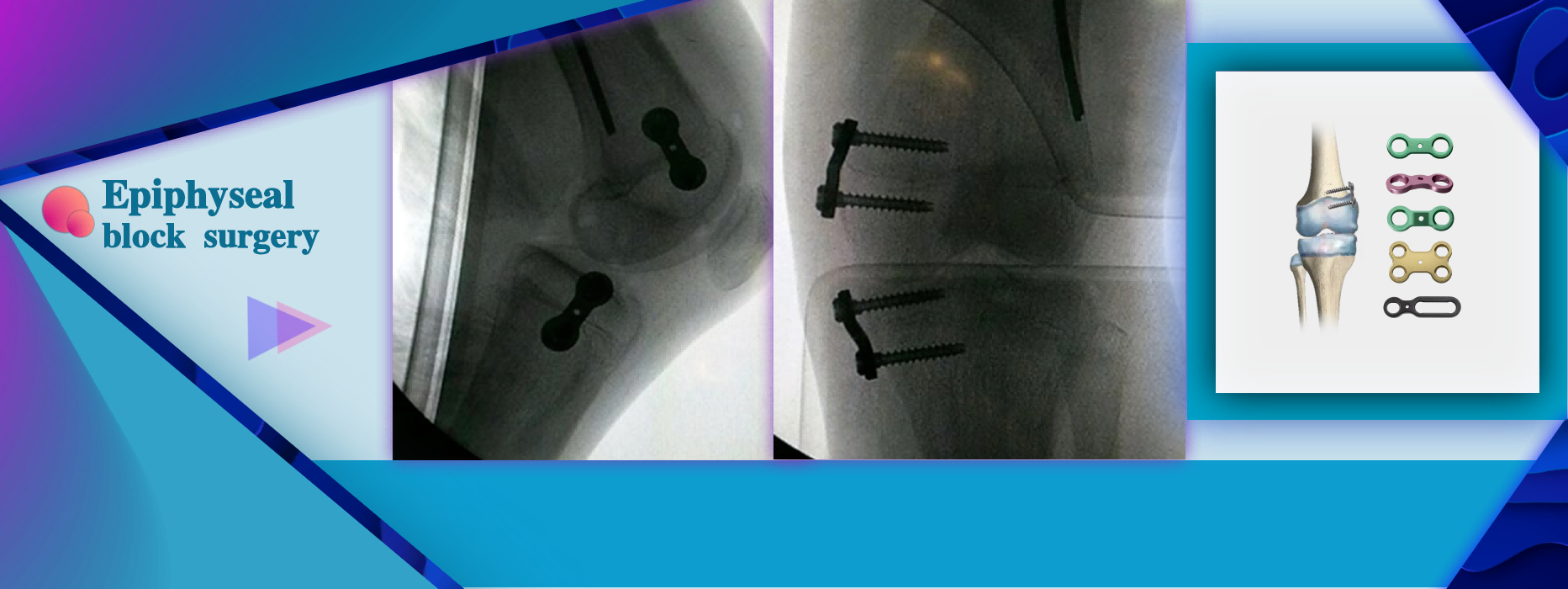
More InformationLimb angular deformity and the need for correction during the growth period of children.
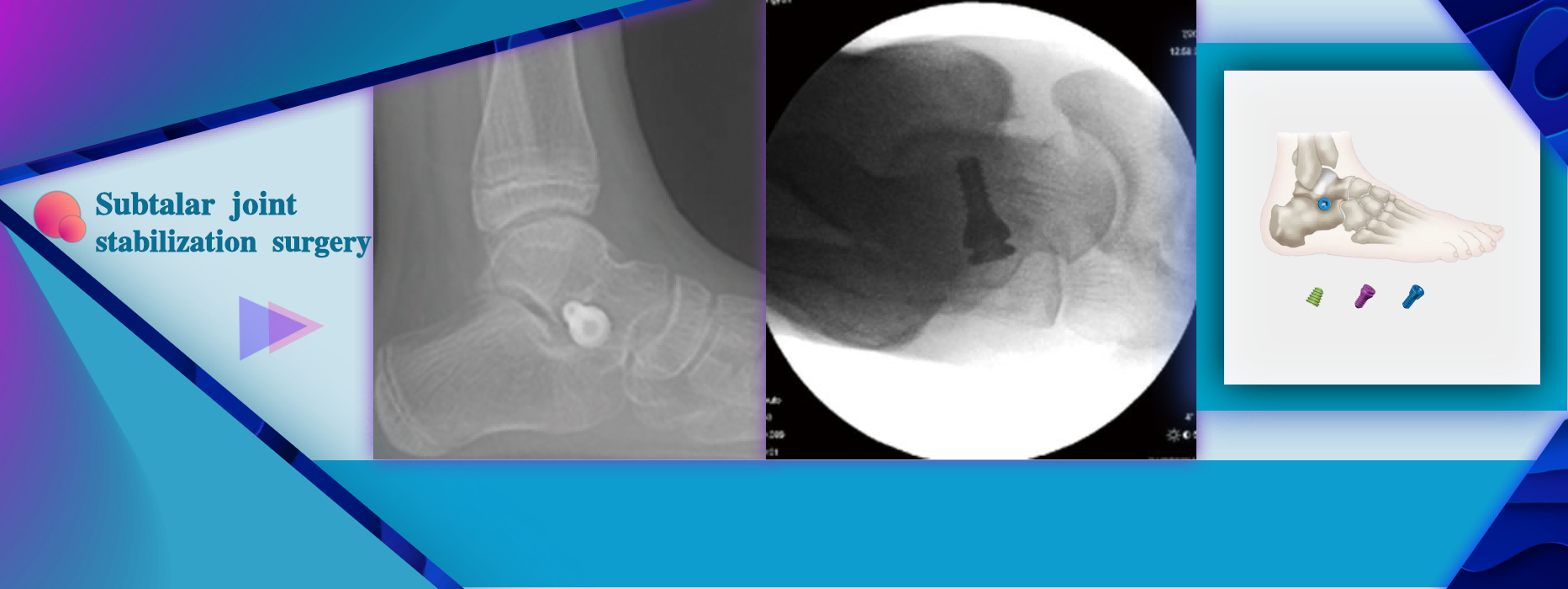
More InformationFlexible flat feet (children, adolescents, adults) Merge flat feet with navicular bone disease Spasmodic paralysis type flat foot (dysfunction of the posterior muscle tendon) Valgus deformity of hind foot Dislocation or instability of the subtalar joint
More Information
Limb extension surgery Limb reconstruction surgery Bone deformity correction surgery
More Information
Limb lengthening surgery Fracture repair surgery Limb reconstruction surgery
More Information
Diabetic lower limb ischemia (Diabetic foot) Arteriosclerosis Obliterans (ASO) Thromboangiitis Obliferans (TAO)
More Information
Severe foot/ankle deformity Arthrosis of ankle and subtalar joints(Traumatic,Rheumatologic) or psderoartthrosis Instability or bone infects after tumor resection Avascular necrosis of the ankle and subtalar joints Failed total ankle replacement or ankle fusion Distal tibial fracture nonunion Severe deformity secondary to neuromuscular ostero- arthropathy (Charcot's foot)
More Information
Treatment for DDH: Proximal Femoral Osteotomy Indication:Inverted, valgus, and rotational osteotomy or fracture fixation of the proximal femur in children
More Information
Limb angular deformity and the need for correction during the growth period of children.
More Information
Flexible flat feet (children, adolescents, adults) Merge flat feet with navicular bone disease Spasmodic paralysis type flat foot (dysfunction of the posterior muscle tendon) Valgus deformity of hind foot Dislocation or instability of the subtalar joint
More Information

We are a specialized enterprise engaged in the R&D, design, production, sales, and technical services of orthopedic medical devices.
They incorporate biomimetic design, minimally invasive approaches, and surface treatments that promote osseointegration, aligning with international mainstream products.
We can align with hospital procurement procedures. Typically required documents include product registration certificates, manufacturing/distribution licenses, technical specifications, clinical data, and compliance documentation—subject to local regulations.
Experienced clinical engineers or product managers can be arranged to provide on-site support based on hospital and surgical requirements.
All medical devices sold in the Chinese market must adhere to national regulations and mandatory standards for production and quality management. They must comply with the Medical Device Production Quality Management Specifications , require operation under the ISO 13485 system, and obtain a Medical Device Registration Certificate to ensure conformity with product technical requirements.