spine implants manufacturer
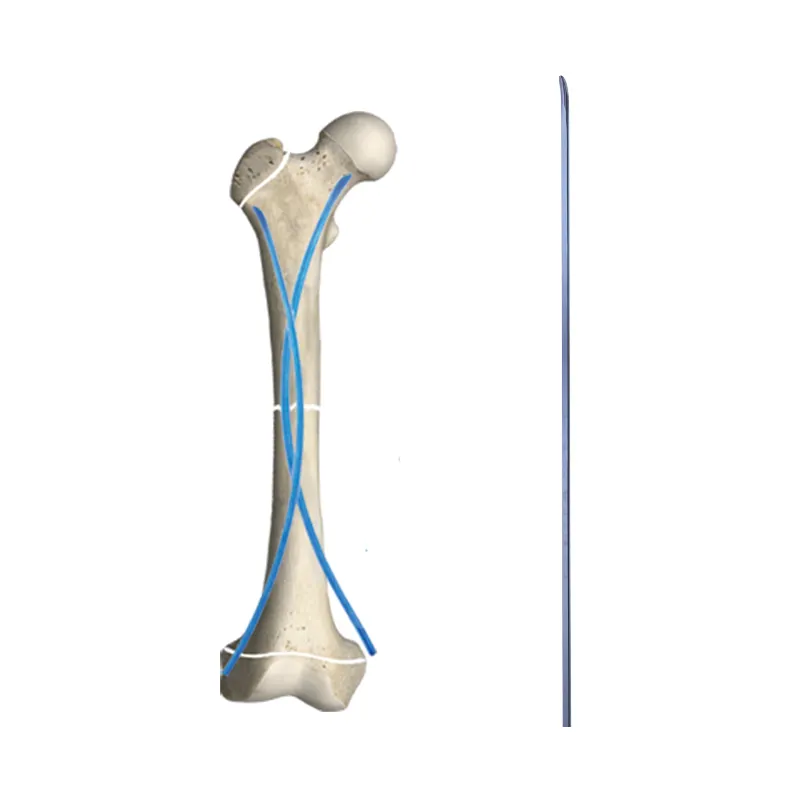
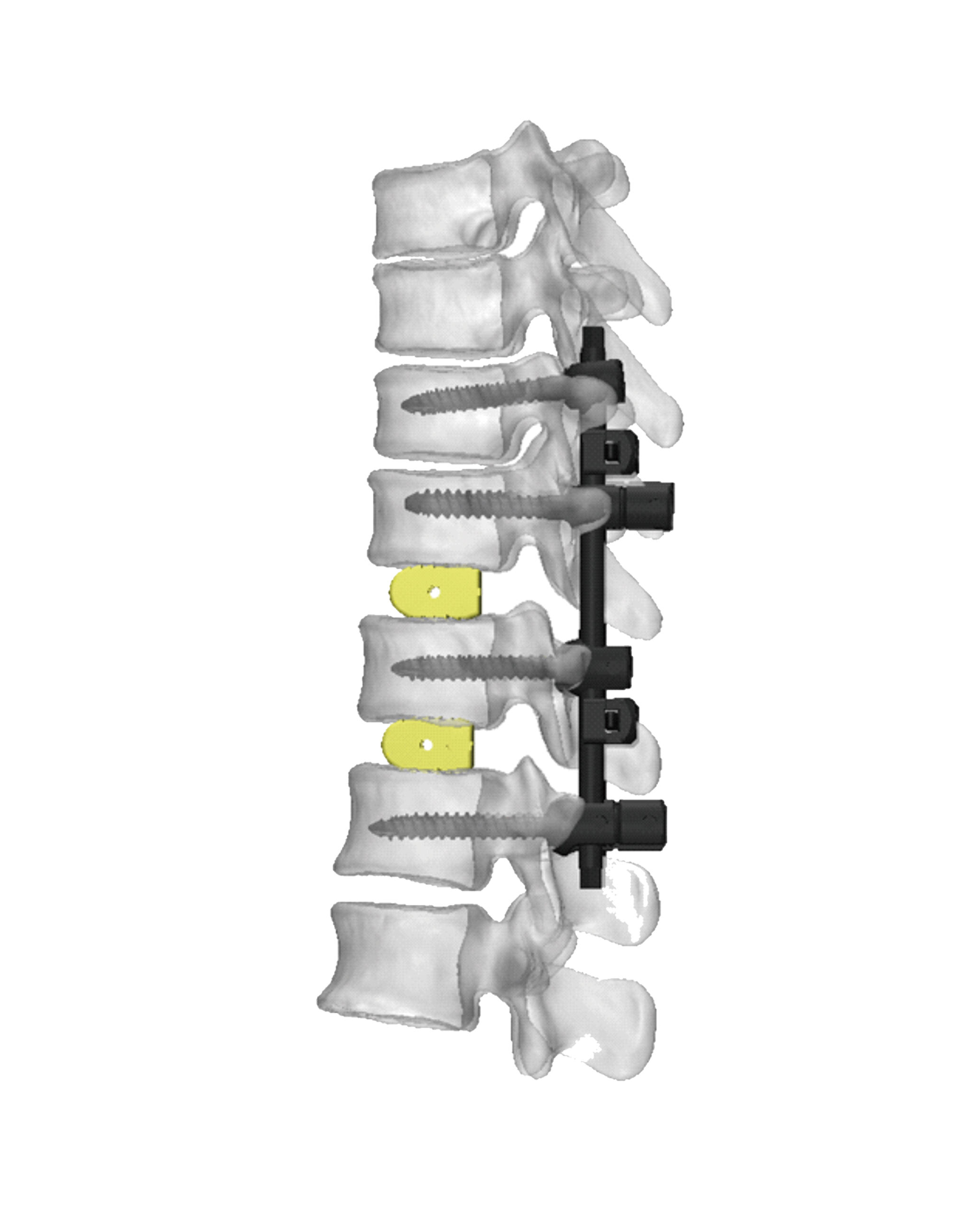
A spine implants manufacturer represents a cutting-edge facility dedicated to developing and producing high-quality medical devices for spinal surgery and treatment. These manufacturers utilize advanced manufacturing technologies and precision engineering to create implants that support various spinal procedures, from basic stabilization to complex reconstructive surgeries. The facility encompasses comprehensive research and development departments, state-of-the-art production lines, and rigorous quality control systems to ensure each implant meets strict medical standards and regulatory requirements. The manufacturing process incorporates advanced materials such as medical-grade titanium, PEEK (polyetheretherketone), and other biocompatible materials that promote optimal patient outcomes. These facilities maintain clean room environments for production, implementing sophisticated tracking systems for complete product traceability. The manufacturer's portfolio typically includes a wide range of products, from cervical plates and pedicle screws to interbody fusion devices and artificial disc replacements. They also provide customization options to meet specific patient needs and surgical requirements. The facility's commitment to innovation is demonstrated through continuous investment in new technologies and regular collaboration with spine surgeons to improve existing products and develop new solutions.