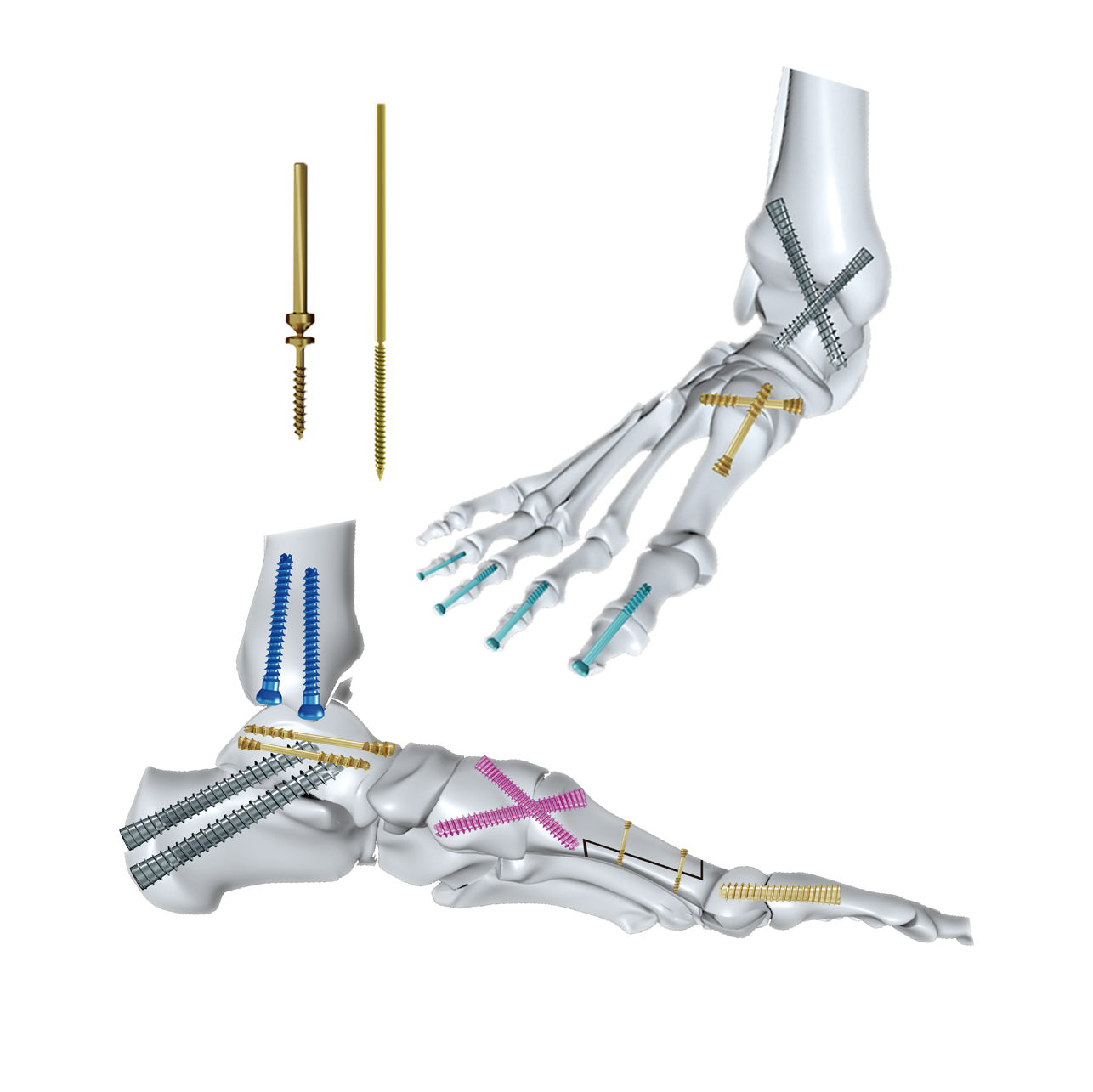
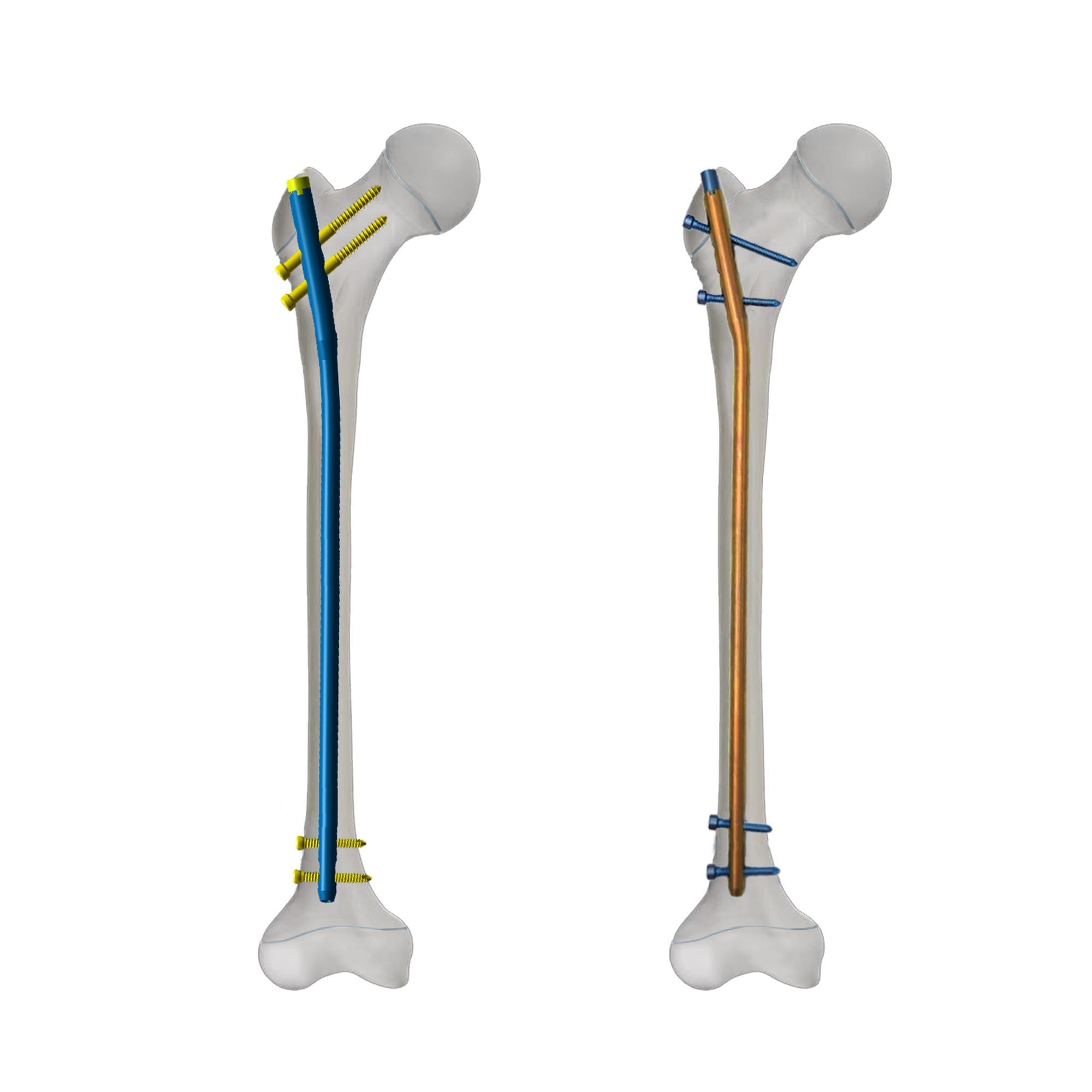
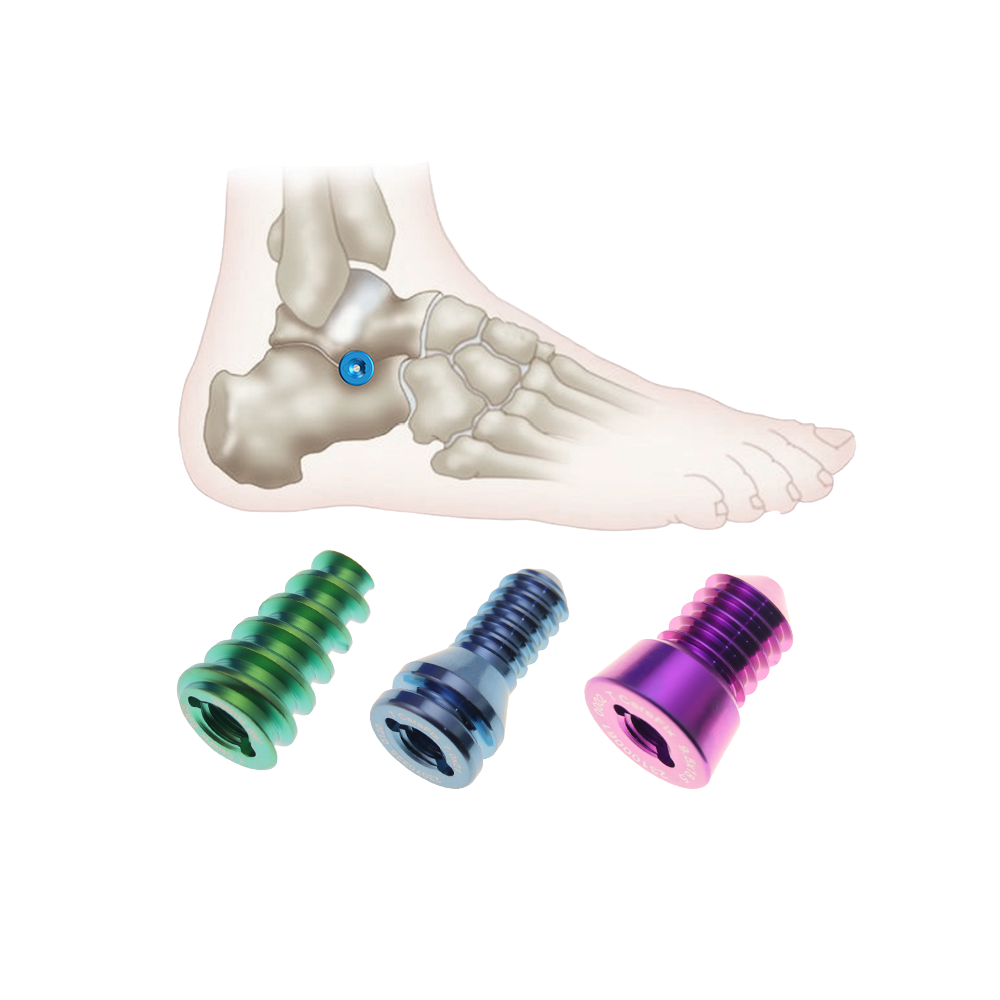
orthopedic implant manufacturer
An orthopedic implant manufacturer stands at the forefront of medical device innovation, specializing in the design, development, and production of high-quality implants for skeletal system restoration. These manufacturers employ cutting-edge technology and precision engineering to create implants that meet rigorous medical standards and improve patient outcomes. Their comprehensive product portfolio typically includes joint replacements, spinal implants, trauma fixation devices, and reconstructive solutions. Using advanced materials like titanium alloys and biocompatible polymers, these manufacturers ensure optimal compatibility with human anatomy. The production facilities maintain strict quality control measures, operating under ISO 13485 certification and FDA guidelines. Modern manufacturing processes incorporate 3D printing technology, computer-aided design (CAD), and automated quality inspection systems to guarantee precision and consistency. These manufacturers also invest heavily in research and development to improve implant longevity, reduce recovery times, and enhance patient mobility. They collaborate closely with orthopedic surgeons and healthcare providers to develop innovative solutions that address specific clinical needs. The manufacturing process integrates sophisticated surface treatment technologies to promote better osseointegration and reduce the risk of complications.