im nail factory
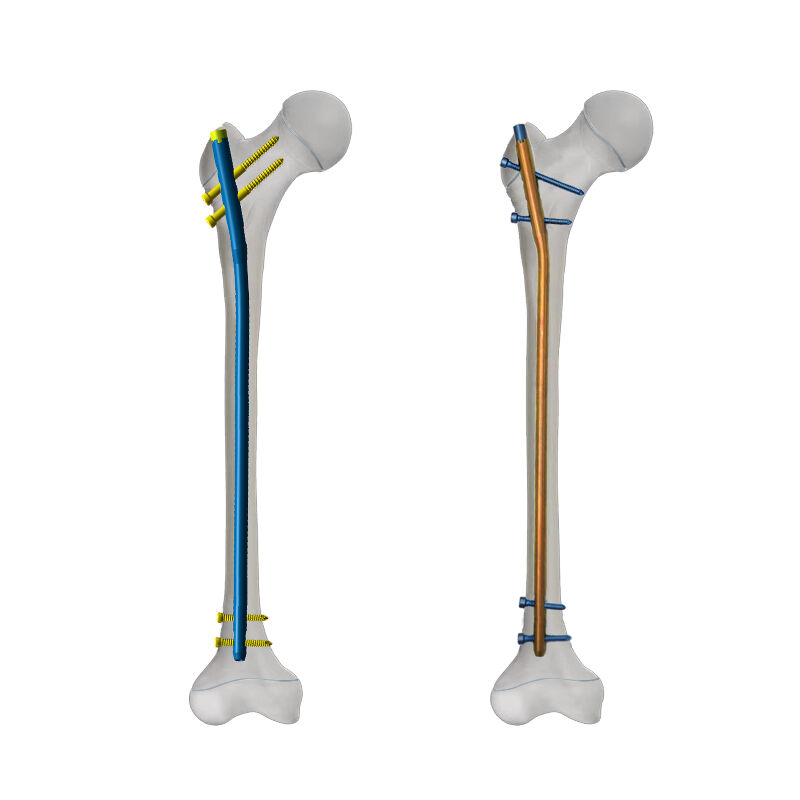
The IM nail factory represents a cutting-edge manufacturing facility specializing in the production of intramedullary nails, essential medical devices used in orthopedic surgery. This state-of-the-art facility integrates advanced automation systems, precision engineering equipment, and rigorous quality control protocols to ensure the consistent production of high-quality implants. The factory utilizes medical-grade titanium and stainless steel materials, processed through sophisticated CNC machining centers and specialized surface treatment facilities. Their manufacturing capabilities encompass a complete range of IM nails, from standard trauma nails to custom-designed solutions for complex fracture patterns. The facility maintains ISO 13485 certification and FDA compliance, operating within clean room environments that meet international standards for medical device manufacturing. The production process incorporates real-time monitoring systems, automated quality inspection stations, and comprehensive documentation procedures to maintain full traceability of each product. With an annual production capacity exceeding 100,000 units, the factory serves global markets while maintaining flexibility to accommodate customized orders and urgent medical requirements.